



The GenAc platform uses synthetic human libraries of antibody fragments (scFv) presented at the surface of filamentous phage particles (G. Winter and G. Smith, 2018 Nobel Prize in Chemistry). Using phage display, scFvs are selected against the antigen of interest presented either as a recombinant protein, peptide or in its physiological environment at the cell surface. After this selection step, the scFvs able to bind to the target are formatted as complete Immunoglobulins and produced in eukaryotic cells. After validation these antibodies can be used directly in your biological tests. In order to facilitate the latter you can choose the immunoglobulin species of interest.
The advantage of this technique is the rapid production of highly specific monoclonal antibodies with good affinity. In addition, the flexibility of the in vitro selection system allows the identification of antibody specificities difficult to obtain by immunization like human-mouse cross-reacting antibodies.
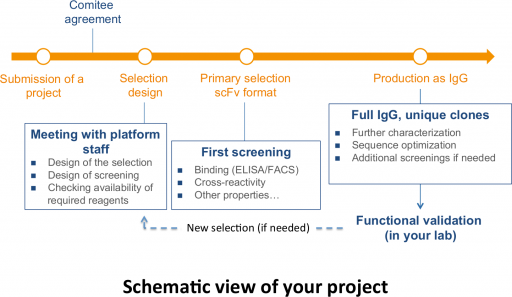
Schematic view of project management
Projects must be submitted to the platform's scientific committee, which will decide on their feasibility. Projects can be submitted via the SIRIC of Montpellier by completing the project form available on the SIRIC genAc website. After validation by the committee, a meeting will be organized to define the selection methods and all the stages of the project. After selection, the platform will provide you with purified IgG for internal validation. If none of the antibodies meets your objectives, a new selection may be considered within the same program. For legal reasons, part of the intellectual property associated with identified antibodies will be allocated to the platform (under the responsibility of Inserm-Transfert).
Developments
In collaboration with the IRCM team Functional screening and targeting in cancer and the RHEM platform, the genAc platform works on the optimization of the selection and production of antibodies
- Production and validation of synthetic human antibody libraries. In addition to the current libraries in scFv format, we are working on the development of Fab libraries
- Optimization of selections, particularly on living cells, by surface display and for IHC applications on fixed and embedded paraffin tissues
- Optimization of clone affinity and specificity by surface display in mammalian cells
- Optimization of antibody production in HEK293 and CHO cells
Patents
METHODS FOR PRODUCING ACTIVE scFv ANTIBODIES AND LIBRARIES THEREFOR. CNRS/Inserm/Université. P. Martineau & E. Weiss. March 2007. EP2134841A2 / US20090143247 / US20100167957.
HUMAN MONOCLONAL ANTIBODIES AGAINST AXL. B. Robert, C. Larbouret, MA Poul & P. Martineau. 8 Dec 2015. WO2016091891A1
HUMAN MONOCLONAL ANTIBODIES AGAINST OREXIN RECEPTOR TYPE 1. A. Couvineau, B. Robert & P. Martineau. 16 Jan 2015. WO2016113351A1 & WO2016113356A1
HUMAN MONOCLONAL ANTIBODIES FRAGMENTS INHIBITING BOTH THE CATH-D CATALYTIC ACTIVITY AND ITS BINDING TO THE LRP1 RECEPTOR. E. Liaudet-Coopman, T. Chardes, P. Martineau, Y. Ashraf. WO2016188911
MONOCLONAL ANTIBODIES BINDING TO THE CD160 TRANSMEMBRANE ISOFORM. A. Bensussan, B. Robert, P. Martineau, M. Chentouf, A. Marie-Cardine, J. Gustiniani. WO2018077926A1